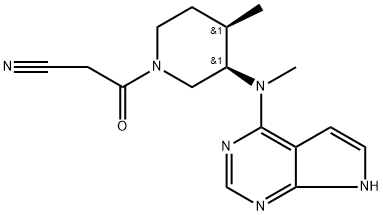
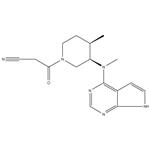
Tofacitinib: pharmacokinetics, pharmacology and safety
![Article illustration]()
![Article illustration]() General Description
General Description
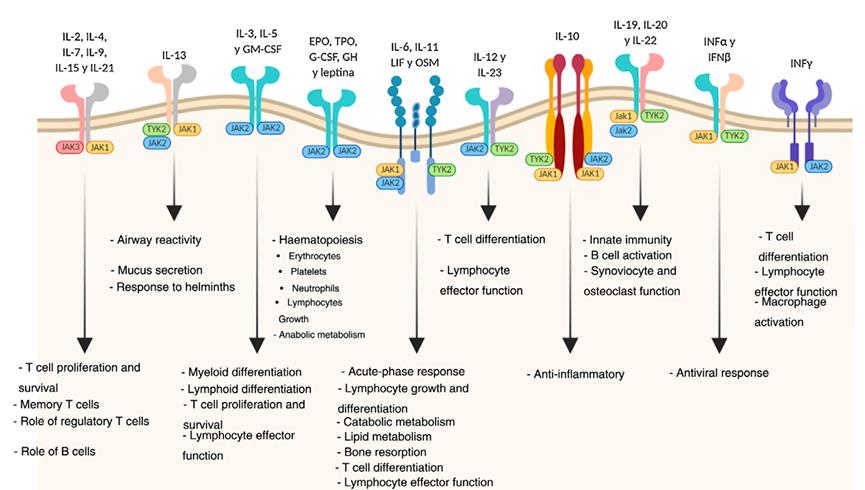
The use of Janus kinase inhibitors is a new approach in the therapy of inflammatory diseases with immune base. Tofacitinib is one of these inhibitors targeting JAK1 and JAK3, and its efficacy has been demonstrated in the treatment of moderate to severe ulcerative colitis and rheumatoid arthritis. It is a small synthetic molecule administered orally, with a fast bioavailability and elimination rate, predictable pharmacokinetics and lack of immunogenicity, which are convenient characteristics for both efficacy and safety. 1

Figure 1. 5 mg tablet of tofacitinib
Pharmacokinetics
Absorption and Distribution
One of the advantages of tofacitinib is its oral administration; it resists gastric degradation and can quickly enter the systemic circulation. Tofacitinib is characterized by good absorption, with or without food (with a bioavailability of 74%), a rapid onset of its effect (peak plasma concentrations are reached within 30 to 60 min), and an increase in systemic exposure proportional to the dose. Stability of plasma concentrations is reached within 24-48 h, with insignificant accumulation after twice daily dosing (2 times/day). There appears to be no influence of age, sex, body weight (no weight-adjusted dosing required), or initial disease severity on the average plasma concentration of tofacitinib20. Tofacitinib moderately binds to plasma proteins, especially to albumin, and in healthy individuals, does not affect glomerular filtration, renal plasma flow, or creatinine clearance. 2, 3
Metabolism and elimination
The elimination of tofacitinib is rapid (the half-life is about 3 h) and studies in healthy volunteers indicate that 95% of the drug (up to 100 mg, single dose) is eliminated within 24 h. In case of need for suspension due to adverse effects or situations such as pregnancy or surgery, this is an advantage because its clearance is fast. Tofacitinib is eliminated 70% by hepatic metabolism and 30% by renal excretion. Its metabolism is mainly mediated by CYP3A4, with a minor contribution from CYP2C1910. It is metabolized into at least 8 metabolites, but the pharmacological activity is attributed to the original molecule. 3
Pharmacology
![Article illustration]()
![Article illustration]()
![Article illustration]() Ulcerative colitis
Ulcerative colitis
Tofacitinib is a small molecule JAK inhibitor recently approved by the FDA for moderate to severe UC induction and maintenance therapy. Although the data on its efficacy as an induction therapy for hospitalized ASUC patients is limited, several factors make it an attractive candidate. It is rapidly absorbed and produces quick clinical improvements in outpatient UC, with a short half-life that minimizes intra and postoperative complications. Compared to biologics, it is less affected by drug loss associated with hypoalbuminemia and colonic protein loss. For patients who have failed infliximab therapy and require alternative inpatient treatment options, tofacitinib is now readily available. Lastly, inpatient tofacitinib is relatively inexpensive when compared to biologic therapy administration. 4
![Article illustration]() Rheumatoid arthritis
Rheumatoid arthritis
In the EU, it is indicated for the treatment of moderate to severe active rheumatoid arthritis (RA) in adult patients who have not responded adequately to, or cannot tolerate, one or more DMARDs. Clinical studies of up to 24 months showed that tofacitinib monotherapy or combination therapy with a synthetic DMARD was effective in reducing RA symptoms and improving quality of life, with benefits lasting for up to 96 months. Tofacitinib also inhibited joint damage progression in methotrexate-naïve patients. The drug was generally well-tolerated, with infections being the most common adverse event. However, the incidence of herpes zoster infections was higher with tofacitinib than in the general RA population. When added to background methotrexate, tofacitinib was noninferior to adalimumab in terms of efficacy and tolerability. While further comparative studies are needed, current evidence supports tofacitinib as a useful option for treating RA patients. 5
Safety
The safety of tofacitinib for patients was studied in a phase 2 clinical trial and several phase 3 studies. The analyzed cohorts included induction and maintenance periods, as well as an extension study. Overall, 1,157 patients were included with a follow-up duration of 6.1 years. Generally, both doses had an acceptable safety profile and were comparable to biologic drugs based on various meta-analyses. Adverse events and serious adverse events were similar across all treatment groups, and the most common side effects were headache, nasopharyngitis, nausea, and arthralgia. Gastrointestinal disorders and infections were the most frequent categories of serious adverse events, and deaths occurred in only 5 patients, with an incidence rate of 0.2 per 100 patient-years of exposure. 1
![Article illustration]()
![Article illustration]() Reference
Reference
1. López-Sanromán A, Esplugues JV, Domènech E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol Hepatol, 2021, 44(1):39-48.
2. Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut, 2017, 66(2):199-209.
3. Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos, 2014. 42:759-773.
4. Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for Biologic-Experienced Hospitalized Patients With Acute Severe Ulcerative Colitis: A Retrospective Case-Control Study. Clin Gastroenterol Hepatol, 2021, 19(10):2112-2120.
5. Dhillon S. Tofacitinib: A Review in Rheumatoid Arthritis. Drugs, 2017, 77(18):1987-2001.
);You may like
Related articles And Qustion
See also
Lastest Price from Tofacitinib manufacturers

US $15.00/kg2024-04-24
- CAS:
- 477600-75-2
- Min. Order:
- 1kg
- Purity:
- 99.912%
- Supply Ability:
- 10ton
US $5.00-0.10/KG2024-04-16
- CAS:
- 477600-75-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available